To evaluate how genetics affect PIBD development, we performed genome wide association study (GWAS) in collaboration with Dr. Yan Zhang also from GWCMC.
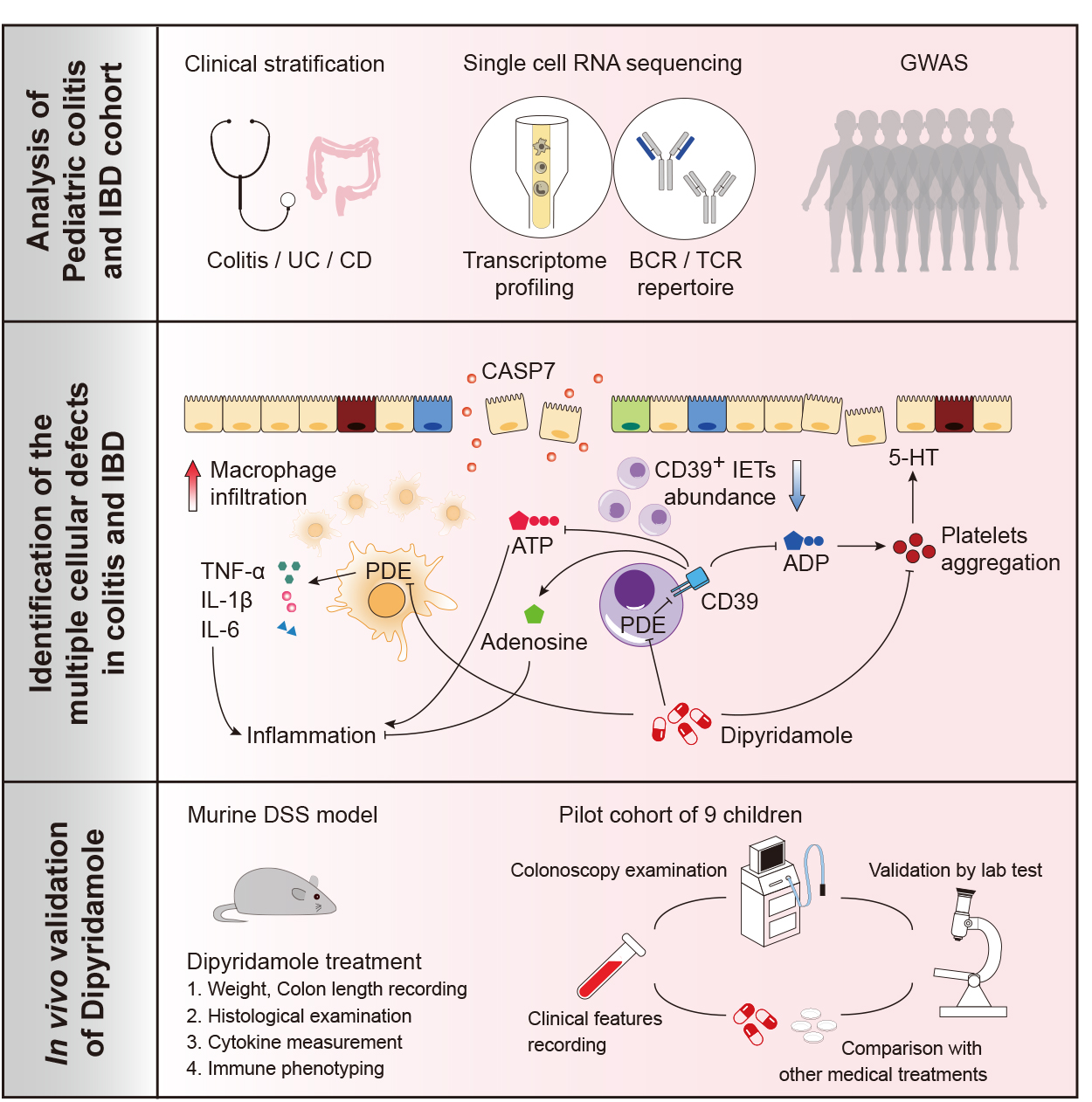
Pediatric onset colitis and inflammatory bowel disease (IBD) represent significant challenges to many infants, children and their guardians. Disease etiopathogenesis remains incompletely understood, and up to 50% children do not respond effectively to conventional therapy (JAMA Pediatr. 2015 Nov;169(11):1053-60. doi: 10.1001/jamapediatrics.2015.1982.).In collaboration with Prof. Fan Bai from Peking University, we performed scRNA sequencing and immune profiling on the colonic mucosae and discovered deficiency of CD39-expressing intraepithelial T cells (CD39+ IETs) in children with colitis or IBD. We hypothesized that this deficiency may lead to accumulation of ATP and ADP, which will subsequently escalate colonic inflammation through activation of macrophages and platelets, respectively. Indeed, platelet dysfunction has long been recognized and perused in the field of IBD (Inflamm Bowel Dis. 2009 Aug;15(8):1245-55. doi: 10.1002/ibd.20896.) or drug-induced anaphylaxis (Sci Immunol. 2018 Apr 13;3(22). pii: eaan5997. doi: 10.1126/sciimmunol.aan5997.).
Strikingly, bioinformatic analysis and functional validation have identified that the multiple immune defects have a common underlying pathogenic mechanism, namely the defective cAMP response pathway. This has led to a direct bench to bed translation and has confirmed that the pan-phoshphodiesterase inhibitor dipyridamole can restore colonic immune homeostasis in a pilot trial involving 9 children with colitis led by Dr. Min Yang from Department of Gastroenterology, GWCMC (http://www.chictr.org.cn/showprojen.aspx?proj=25986). We will continue to explore treatment efficacy of dipyridamole in PIBD subtypes, identify biomarkers that are predictive of responsiveness, and test whether other specific PDE inhibitors maybe more effective in the treatment of IBD.
To evaluate how genetics affect PIBD development, we performed genome wide association study (GWAS) in collaboration with Dr. Yan Zhang also from GWCMC. This has identified novel disease risk genes, such as DVL1, CASP7, PLEZO1 that affect differentiation and function of epithelial, fibroblast or immune cells in the colonic mucosae. Future studies are ensued to study how these risk genes and disease-causal genes (that we have identified from whole exome sequencing of the patients and their parents) increase disease risk in children.
Yuxia Zhang
28 June 2021